do you need to autoclave potassium acetate|STOCK SOLUTIONS : advice Potassium acetate is frequently used in the preparation of plasmid DNA through the aid of . AUTOCLAVE CYCLES Virginia Tech EHSS Form (04-08) Autoclave Cycles CYCLE TYPE: RECOMMENDED FOR:
{plog:ftitle_list}
on both solid and hollow instruments. Reliable For over 25 years, STATIM’s unmatched speed, reliability, and dependability have made it one of the world’s best selling cassette autoclaves. .During brewing, your beer is often accidentally exposed to bacteria not killed by typical sanitizing methods. Brewing sanitizer is less effective than disinfectant, which is, in turn, less effective than sterilization. Any bacteria that survives the cleaning process can make your beer taste musty, vinegary, and even . See more
To prepare 1 liter of this solution, dissolve 294.42 g potassium acetate in 100 ml water, and add glacial acetic acid until a pH of 4.6 is reached. This will require about 40-50% of the final volume to be acetic acid.
Potassium acetate is frequently used in the preparation of plasmid DNA through the aid of . 60 mL of 5 M potassium acetate, 11.5 mL glacial acetic acid, 28.5 mL double-distilled H2O. The resulting solution is 3 M acetate and 5 M potassium and has a pH of about 4.8. Store at room temperature, and do not autoclave Boiling Lysis Method (reference here) This procedure is quick and reliable for preparing plasmid from small volume cultures.Since sodium acetate is a salt, its addition enables the precipitation of nucleic acids out of solution. Download the recipe as a PDF. To download the 3 M sodium acetate recipe as a PDF then click here. 3 M sodium acetate, pH 5.2 recipe. The recipe below can be used to prepare a 100 mL 3 M sodium acetate, pH 5.2 solution.Add 17.67 g potassium acetate (if mixing yourself: 0.18mol = 10.09 g KOH dissolved in water + 10.809 ml glacial acetic acid). Adjust the pH to 4.8 with glacial acetic acid. Make up to 200 ml with autoclaved dH2O. Don’t need to sterilise. See video for 100 ml and 1 L recipes, safety instructions and much, much more;
The molecular mass of zinc acetate dihydrate is 219.5. Thus to prepare a 0.02 M solution in DI water requires 219.5*0.02 = 4.39 g/L. In the article 50 mL is mentioned as the quantity.You can not find a comprehensive list of those chemicals/compounds/ liquids etc, which can not be autoclaved. but you can try to prepare a list on the bases of answers which you will be getting .
It depends a bit at the final use and how much do you need, if you need liters it is a pain to squeeze through a filter but just a few cl is ok, make sure you filter into a sterile receptacle .which buffer need to autoclave, which can not - (reply: 1) preparation of solutions that need pH adjustment - (reply: 3) NP40 concentration in protein lysis buffer - (reply: 1) Balanced salt solutions - (reply: 2) smear of loading buffer observed - SDS-PAGE gel (reply: 5) How do you prepare PCR stock solution to minimize pipetting - (reply: 37)
You have to dissolve 1.00 mol, that is 98.15 g CH3COOK (its molar mass being 98.15 g/mol), in upto 1.000 L.(Suggested procedure: dissolve 98.15 g CH3COOK in not more then 900 mL, homogenize and .
Potassium acetate buffer, 0.1 M. Potassium phosphate buffer, 0.1 M. Saponin, 10% (w/v) . If you do not receive an email within 10 minutes, your email address may not be registered, and you may need to create a new Wiley Online Library account. Request Username. Can't sign in? Forgot your username?a. You need to make an aqueous solution of 0.221 M potassium acetate for an experiment in lab, using a 250 mL volumetric flask. How much solid potassium acetate should you add?. b. How many milliliters of an aqueous solution of 0.126 M sodium nitrate is needed to obtain 7.14 grams of the salt?. c. In the laboratory you dissolve 17.1 g of potassium sulfate in a volumetric flask and .Potassium acetate buffer, 0.1 M. Potassium phosphate buffer, 0.1 M. Saponin, 10% (w/v) . If you do not receive an email within 10 minutes, your email address may not be registered, and you may need to create a new Wiley Online Library account. Request Username. Can't sign in? Forgot your username?
You need to make an aqueous solution of 0.228 M potassium hydroxide for an experiment in lab, using a 125 ml volumetric flask. How much solid potassium hydroxide should you add? grams How many milliliters of an aqueous solution of 0.248 M copper(IT) bromide is needed to obtain 9.07 grams of the salt? ml In the laboratory you dissolve 24.4 g of calcium acetate in a .I don't ahaha. When you autoclave them you are just making them dirtier. All water vapour going everywhere and yes I know it should be distilled water but have you ever smelled an autoclave? add. I think a good way of understanding why people do certain things is seeing how flawed you are. Everyone is flawed and everyone does stupid things.I autoclave 1 M Imidazole at pH 8, but nothing else. Some buffers decompose in the autoclave. After about 2 months, both MES and MOPS buffer turn yellow at room temperature by oxidation. The same thing happens in the autoclave. Yellow MES and MOPS is an oxidized form of the buffers. Nobody knows what it is. Nobody cares. The pH remains the same .Answer to 1. You need to make an aqueous solution of 0.132 M. 1. You need to make an aqueous solution of 0.132 M potassium sulfate for an experiment in lab, using a 250 mL volumetric flask. How much solid potassium sulfate should you add? ____ grams. 2. In the laboratory you dissolve 21.2 g of sodium chloride in a volumetric flask and add water to a total volume of 250 . mL.
Question 1 A) You need to make an aqueous solution of 0.225 M potassium chloride for an experiment in lab, using a 125 mL volumetric flask. How much solid potassium chloride should you add? grams B) How many milliliters of an aqueous solution of 0.140 M manganese(II) chloride is needed to obtain 5.55 grams of the salt? mL C) In the laboratory you dissolve 17.5 g of silver . Hi all, i have a doubt, when you prepare 3 M sodium acetate in certain volume the sodium acetate increase the volume for example, if i need to prepare 100 ml of this solution, i need to add 40.83g of NaC2H302 and then when i add 50 the water, the volume increase almost twice , so.. is necesary to add the rest of the water ?????, what is the real concentration de .E.coli broth excess or sterilized that can go down the sink, along with excess agar (THAT CAN GO IN THE TRASH), AS LONG AS THERE ARE NO ACTIVE LIVE CULTURES IN IT THEN WHO CARES, IF YOU SUSPECT THERE IS A CULTURE THEN AUTOCLAVE IT AND DO NOT TRASH IT but give it to somebody for proper disposal, They may even do the autoclaving . You need to prepare an acetate buffer of pH 6.32 from a 0.800 M acetic acid solution and a 2.46 M #KOH# solution. If you have 925 mL of the acetic acid solution, how many milliliters of the #KOH# do you need to add to make a bufer of .
you'll need: g kanamycin: freeze. Kan calcs Kan calcs kdorfman Tue, 10/02/2012 - 16:23. if Kanamycin stock is: . Do not autoclave! Cobalt Chloride Cobalt Chloride kdorfman Tue, 12/20/2011 . Potassium acetate (C2H3O2K) Fisher BP364-500 .30. MW = 98.14. 49 g in 100 mL H2O. MW of KOAc: Concentration: M:Two-Phase Reaction of 1-Bromooctane with Sodium Acetate and Potassium Acetate Catalyzed by Diquaternary Ammonium Salts New unsaturated diquaternary ammonium salts (diquats) were prepared in two .
Autoclave the solution to inactivate the remaining DEPC. CAUTION: Wear gloves and use a fume hood when using DEPC, as it is a suspected carcinogen. Many investigators keep the solutions they use for RNA work separate to ensure that fidirtyfl pipets do not go into them. Do not treat solutions containing Tris with DEPC, as Tris inactivates the . How Does It Work? An autoclave uses heated, pressurized steam to kill off all microbial forms of life on equipment placed inside it – bacteria, viruses, fungi, you name it.. There are several types of autoclaves used in different industries. An autoclave found in cosmetic salons is usually a smaller device that looks a lot like a microwave.. It has a chamber where you put .Its a very routine process in our lab. We never purchase the solution. You need to know the molarity of the solution before going into the details. Lets consider it 1M, pH 9 and you need to .How many grams do you need for 800 ml of 400 µM potassium acetate? The molecular weight of potassium acetate is 98.14grams/mol. Here’s the best way to solve it.
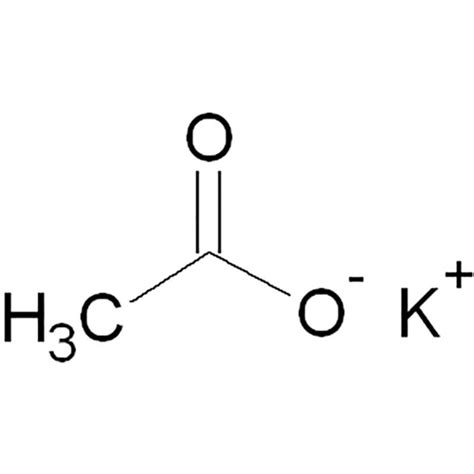
Question: You need to make an aqueous solution of 0.171 M potassium acetate for an experiment in lab, using a 250 mL volumetric flask. much solid potassium acetate should you add? grams Show transcribed image text
STOCK SOLUTIONS
how does a honey refractometer work
Steam sterilization (autoclaving) is the most widely used method for sterilization and is considered the most robust and cost-effective method for sterilization of medical .
do you need to autoclave potassium acetate|STOCK SOLUTIONS